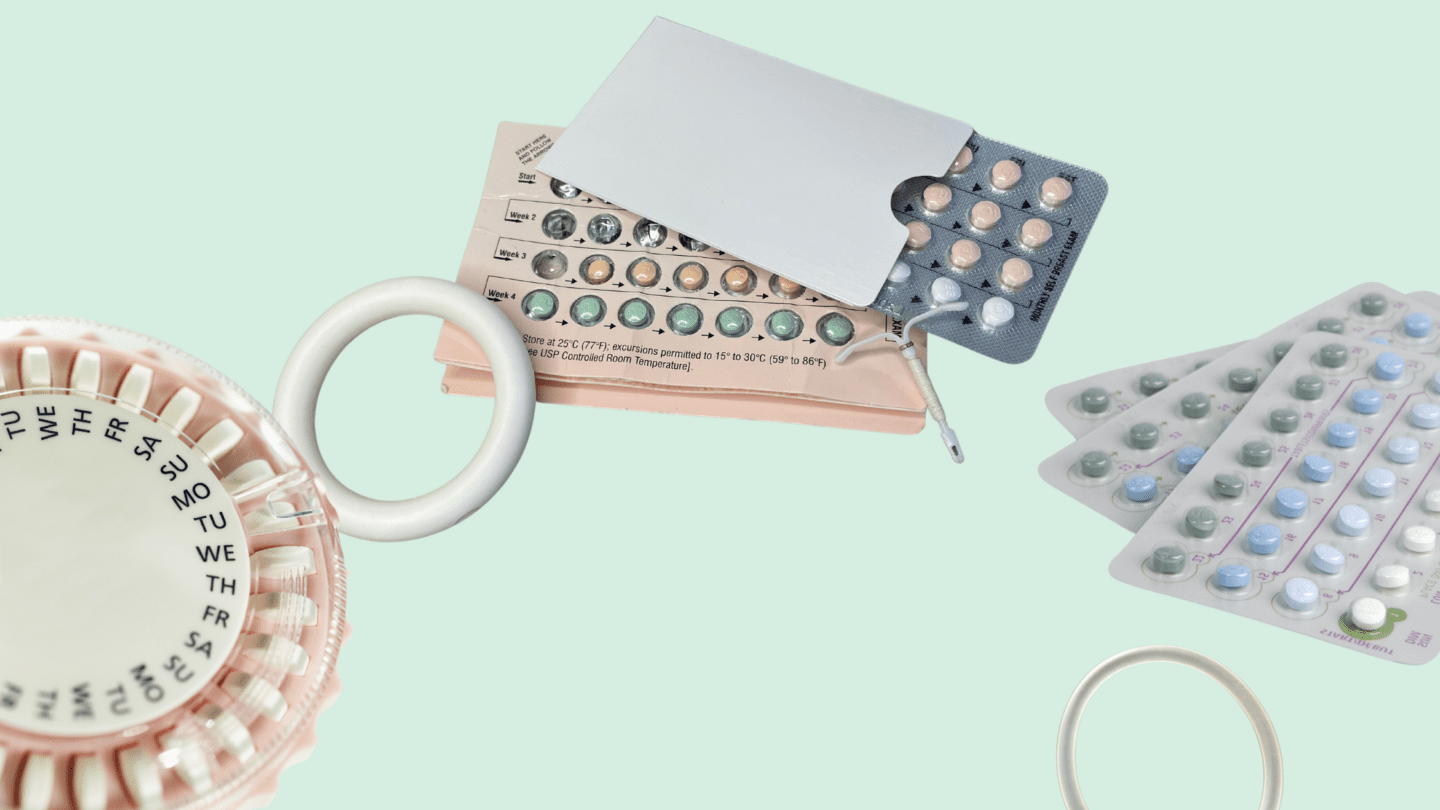
Brand Name vs. Generic
When you have a headache, do you reach for Advil or ibuprofen? It’s a bit of a trick question: Advil is just the brand-name version of ibuprofen, and they both contain the same active ingredient that helps reduce pain. There are generic versions of drugs for almost every brand-name medication out there.
Hormonal is no different: Loryna and Melamisa1 are generic versions of Yaz, Tri-Sprintec is a generic for Ortho Tri Cyclen,2 Tri-Legest Fe 28 is a generic version of Estrostep Fe,3 and so on for most branded options you can find. Generics are often far cheaper than brand-name drugs, and they typically do exactly the same thing.
Except, in some cases, they don’t. While there haven’t been robust studies of generic versus brand-name birth control pills, anecdotal evidence and studies of other generic drugs indicate that some people react differently to brand vs. generic versions of . And when insurance plans may only cover one version of a medication, that can leave people struggling to find that’s right for them.
So how did we wind up in this situation in the first place? And, crucially, if you’re stuck with a version of that doesn’t work for you, what can you do about it?
What’s a generic drug?
Generics are drugs that have the same active chemical compounds in the same amounts as the brand name drug, but are sold under different names and in different packaging.4 So, for a combined birth control pill, the generic version would theoretically have the exact same amount of and as the brand name option — though in practice there can be slight variations. A generic drug must also be taken the same way as the brand name equivalent — so if the brand name is a pill, the generic needs to be a pill too.4
Not every part of a generic drug is exactly the same as the brand name, though. Many generics have additives like colors, binding agents, flavorings and other things that are different from the brand name version.4 That won’t affect how the drug works, but in some cases it might change the side effects you experience.
Not every part of a generic drug is exactly the same as the brand name
The FDA also allows for some variation when it comes to active ingredients in generics. Their rules allow the actual amount of active ingredient absorbed into the body to vary slightly in different versions of drugs and even between batches of drugs. That variation is typically around four percent of the amount that’s in the brand name, according to a large review study comparing generic and brand name drugs.5-6
To get approved, generic drug manufacturers must prove that their products have the same effects as brand name drugs. They submit data to the FDA showing that their active ingredients are pharmaceutically equivalent to the brand name drugs, that they can make the drug correctly and consistently, that the label is correct, and more.7 But because they use the exact same active ingredients as brand name drugs, generics don’t need to go through the multiple rounds of animal and human clinical trials that brand name drugs do to be initially cleared for use — a big factor in lowering costs.
Why are generic drugs cheaper?
When a pharmaceutical company wants to get a new drug approved, it needs to go through multiple rounds of clinical trials to prove that the drug is safe and effective.8 And that’s on top of the costs of developing the drug in the first place. All told, it means that making a drug is expensive and time-intensive. That’s not always an excuse for sky-high drug prices, of course, but it does mean that brand name drugs will typically be a lot more expensive than their generic counterparts.
Like what you’re reading? Get the latest straight to your inbox 💌
Pharmaceutical companies will usually patent their drugs and, on top of that, they’ll be granted what are called “exclusivities” that give them the right to make and market a drug without any competition. The length of these exclusivities varies, but it can be five years or more.9
The typical rationale for these exclusivities is so that pharmaceutical companies can recoup the costs of making a drug and getting it approved. But once that exclusivity period is over, generic drug makers can step in and sell their own versions of a drug.
Those generics can make a big difference for the people buying the drugs. According to the FDA, just one generic drug entering the market can lower prices by 30%, and five competing generics can lower prices by as much as 85%.10 In 2019 alone, generic drugs saved Americans $313 billion, according to a report by the Association for Accessible Medicines, a generic drug industry trade association.11 And 90% of prescriptions in the U.S. are for generic drugs, says the FDA.12
There are generic options for many kinds of out there, from the pill to the vaginal ring. And the lower costs generics bring can make a big difference for people using , too. In a 2016 study, 71 percent of respondents said it was important that their was low cost.13 And another study found that higher prices for IUDs were associated with lower IUD use, implying that cost might be preventing people from trying out a different kind of .14
Do generic drugs have different side effects?
While generic drugs need to have the same active ingredient as brand name drugs, they’re also required to look different, to avoid trademark infringements.4 That means that generics will have different non-active ingredients, like dyes for coloring, than brand name drugs, and those ingredients could cause new side effects. This isn’t a given, of course, and the opposite is just as likely: A generic drug could not have a side effect that a brand name drug does have. Still, it’s something to watch out for.
It’s also important to remember that the amount of active ingredient in a generic drug that’s absorbed by your body can differ slightly from its branded counterpart. That disparity could also lead to variations in side effects.
There’s anecdotal evidence that switching between brands of can change the side effects you feel. For example, an NPR article from 2021 details how switching from the NuvaRing to a generic vaginal ring caused one woman to develop headaches and feel irritable and short-tempered.
But actual studies comparing the side effects of brand-name and generic options don’t exist — yet another glaring example of inequities in medical research. For some general context, we can look at research that followed people who switched from brand-name to generic versions of other sorts of drugs. For example, one review study found that patients in multiple studies reported new side effects when switching to generic versions of drugs.15 And Canadian researchers found an increase in hospitalizations for cardiovascular issues immediately after three generic versions of heart disease drugs were approved — though the authors do caution that other factors could have been involved, as well.16
Overall, these studies don’t necessarily show that generic drugs are worse, but simply that they can sometimes be different. The same goes for : Different people may react differently to generic versions. For some, the brand name option might feel better, while a generic might work best for others. And we should note that generic is just as effective as brand name when it comes to preventing pregnancies.
Will my insurance cover the I need?
The Affordable Care Act of 2010 requires most insurance plans nationwide to provide cost-free to everyone (some plans or workplaces may be able to file exemptions for religious or moral reasons). Some states have additional requirements as well, like mandating that plans must allow people to get over-the-counter methods or get an extended supply of in advance.17 Health insurers can’t favor one method over another (say, preferring an IUD over the vaginal ring), and they need to offer at least one of each of the 18 federally-recognized methods for women.17 That list includes options like the pill, implant, patch, ring and IUDs, though it does not include vasectomies or male condoms.18
Is adyn right for you? Take the quiz.
But some people still face an uphill battle getting the best for themselves. Because generic and brand-name versions of can bring different side effects, some people need different versions. Although research is lacking, we do know that people react differently to different versions of the combined pill, as well as other kinds of . Some people may experience heavy bleeding, cramps, headaches, mood problems, and more on one kind of birth control pill, but not another. But under the ACA currently, an insurance provider only needs to cover one kind of birth control pill to be in compliance. That means it might be difficult to switch from one version of a birth control pill to another and still have your insurance cover it.
Additionally, new kinds of can take a long time to get added to the federal list, meaning insurers may not cover them right away. Some plans also require patients to get approval from the insurance company for certain versions of ,19 a process that can be difficult and confusing. There’s also the fact that generic versions are cheaper, giving insurers a financial incentive to cover just the generic versions of .19
It’s something the American College of Obstetricians and Gynecologists, among other groups, thinks should change. In a 2007 opinion statement, ACOG says that the decision for which version of to use should ultimately rest with the patients and their providers — not insurance companies or legislators.20
“The American College of Obstetricians and Gynecologists supports patient or clinician requests for branded [oral contraceptives] or continuation of the same generic or branded [oral contraceptives] if the request is based on clinical experience or concerns regarding packaging or compliance, or if the branded product is considered a better choice for that individual patient.”
“the decision for which version of to use should ultimately rest with the patients and their providers — not insurance companies or legislators”
If you’re having trouble getting the version of you need, you and your provider can file what’s known as an exceptions policy with your insurer. These little-known claims, sometimes also called waivers, allow a patient to get cost-free coverage of if there’s a medical reason to do so, and insurers are required by the ACA to comply.21 But the process for filing an exceptions policy can differ from state to state, and many patients and doctors may not know about them.
If you need to file an exceptions policy, the best thing to do is talk to your doctor, and then reach out to your state insurance department if you have issues with your insurance company. The National Women’s Law Center’s CoverHer hotline can also provide assistance with dealing with insurance issues.
It can be overwhelming to realize that the differences between brands of and their generics means there are even more options out there. But you should trust yourself when deciding what is right for you — and be ready to advocate for what you need. There are many options for out there, and we promise to give you all the information you need to choose the right one.
Looking for the best for your body? Get started with The Birth Control Test.
-
- Drugs.com. “Generic Yaz Availability.” Drugs.com (2022 May 11): Last accessed 2022 May 31.
- Teva Generics “Generic of Ortho Tri-Cyclen® Tablets.” Tevagenerics.com (N.D.): Last accessed 2022 May 31.
- Teva Generics “Generic of Estrostep® Fe Tablets.” Tevagenerics.com (N.D.): Last accessed 2022 May 31.
- FDA. “Generic Drugs: Questions & Answers.” FDA.gov (2021 Mar 16): Last accessed 2022 May 31.
- Davit, Barbara M., et al. “Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration.” Annals of Pharmacotherapy 43.10 (2009): 1583-1597.
- Andrade, Chittaranjan. “Bioequivalence of generic drugs: a simple explanation for a US Food and Drug Administration requirement.” The Journal of Clinical Psychiatry 76.6 (2015): 1907.
- FDA. “What Is the Approval Process for Generic Drugs?” FDA.gov (2017 Aug 31): Last accessed 2022 May 31.
- FDA. “The FDA’s Drug Review Process: Ensuring Drugs Are Safe and Effective.” FDA.gov (2017 Nov 24): Last accessed 2022 May 31.
- FDA. “Frequently Asked Questions on Patents and Exclusivity.” FDA.gov (2020 Feb 05): Last accessed 2022 May 31.
- FDA. “The Generic Drug Approval Process.” FDA.gov (2022 Mar 17): Last accessed 2022 May 31.
- Association for Accessible Medicines. “Generic Drug & Biosimilars Access and Savings in the U.S. Report.” RxAccessReport.us (2020).
- FDA. “Generic Drugs.” FDA.gov (2021 Feb 05): Last accessed 2022 May 31.
- Johnston, Emily M., Brigette Courtot, and Genevieve M. Kenney. “Access to contraception in 2016 and what it means to women.” Urban Institute (2017).
- Pace, Lydia E., et al. “The impact of out-of-pocket costs on use of intrauterine contraception among women with employer-sponsored insurance.” Medical Care 51.11 (2013): 959.
- Håkonsen, Helle, and Else-Lydia Toverud. “A review of patient perspectives on generics substitution: what are the challenges for optimal drug use.” Generics and Biosimilars Initiative Journal 1.1 (2012): 28-32.
- Leclerc, Jacinthe, et al. “Impact of the commercialization of three generic angiotensin II receptor blockers on adverse events in Quebec, Canada: a population-based time series analysis.” Circulation: Cardiovascular Quality and Outcomes 10.10 (2017): e003891.
- Guttmacher Institute. “Insurance Coverage of Contraceptives.” Guttmacher Institute (2022 May 01).
- Health Resources & Services Administration. “Women’s Preventive Services Guidelines” (2022 Jan): Last accessed 2022 May 31.
- Sobel, Laurie, Adara Beamesderfer, and Alina Salganicoff. “Private insurance coverage of contraception.” The Henry J. Kaiser Family Foundation (2016 Dec 07).
- Committee on Gynecologic Practice. “ACOG Committee Opinion No. 375: Brand versus generic oral contraceptives.” Obstetrics and Gynecology 110.2 Pt 1 (2007): 447-448.
- National Women’s Law Center. “Exception Policies: Advocating for no-cost coverage of noncovered contraceptives.” Issue Brief (2021 Mar).